Audits assist recognize places for enhancement and make sure that testing activities align Together with the Group’s good quality administration technique.
Strategies for examining microbial limits Participate in a vital function in making sure protection and good quality throughout different domains including foods, pharmaceuticals, and environmental monitoring. These approaches permit experts and wellness officers to determine the amounts of microorganisms present in products and involved environments.
This kind of breakthroughs prompted even more investigations into your roles that microorganisms Engage in in human health, illness, and also the setting.
Even so, the distinctive element of these expectations is that they're usually source-intensive to implement, which may be a drawback for smaller businesses missing exactly the same abilities as larger sized enterprises.
Simultaneously, the toxic metabolites of microorganisms and several pathogenic microorganisms could also trigger adverse reactions or secondary bacterial infections to sufferers. Hence, microbial limit testing for non-sterile drug products is probably the important steps to make certain the standard, protection, and success in the medication.
Additionally, it illustrates the societal and scientific requires that have shaped these limits. Recognizing this context makes it possible for researchers and practitioners to understand the necessity of compliance in sustaining public well being and security.
Thus, microbial contamination may end up in bacterial infections or irritations. Manufacturers must equilibrium successful preservation with basic safety and regulatory compliance.
This consists of a radical evaluate of the methods used for microbial limit testing, ensuring they align with regulatory requirements and industry best methods.
Two Major techniques dominate the assessment processes: lifestyle-primarily based methodologies and non-culture-dependent methods. Just about every technique has its special strengths and limitations, which makes it important to know when and the way to use them proficiently.
Microbial Limit website Tests are very important in ensuring the safety and high-quality of Uncooked products and finished products, notably in industries including pharmaceuticals, food items, and cosmetics.
Essential milestones in microbial study have substantially motivated the institution of microbial limits. Notably, the invention of penicillin by Alexander Fleming in 1928 catalyzed a paradigm shift from the idea of bacterial behaviour and resistance.
Encouraging dialogue in between gurus across fields ensures that new results aren't isolated. more info By sharing insights and details, the study Neighborhood builds a reliable foundation for placing microbial limits that truly replicate the complexities of modern science.
As industries grow to be extra reliant on specific microbial Command, these progress keep fantastic promise for strengthening compliance with safety criteria and enhancing public well being safety.
The https:// makes sure that you are connecting for the Formal Web page and that any facts you present is encrypted and transmitted securely.
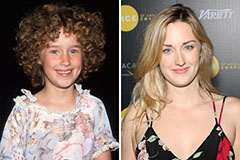 Ashley Johnson Then & Now!
Ashley Johnson Then & Now! Jeremy Miller Then & Now!
Jeremy Miller Then & Now! Macaulay Culkin Then & Now!
Macaulay Culkin Then & Now! Richard Thomas Then & Now!
Richard Thomas Then & Now! Dolly Parton Then & Now!
Dolly Parton Then & Now!